Background: The optimal interruption schedule for NOACs in patients undergoing invasive procedures has not been determined. This analysis describes periprocedural interruption of edoxaban in real world EMIT AF/VTE study.
Methods: EMIT AF/VTE was a prospective, non-interventional study conducted in 7 European countries that collected detailed information on periprocedural management of edoxaban from 5 days before to 30 days after procedure in patients with AF or VTE.
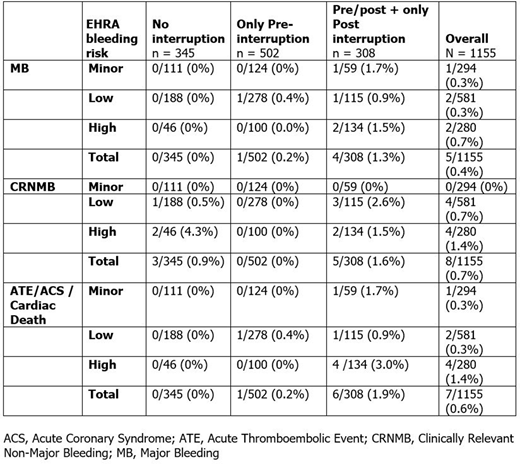
Results: Among 1155 patients, pre-procedural interruption occurred in 781 (68%) patients (median 2 days, IQR 1.0-2.0) and post-procedural interruption in 308 (27%) patients (median 3 days, IQR 1.0-8.0); 279 (24.2%) patients had both pre- and post-procedural interruption. Edoxaban interruption in some patients differed from 2018 EHRA Recommendations for NOAC use in AF. Of 294 patients receiving minor risk procedures, for whom no interruption is recommended, 173 (58.8%) had preprocedural interruption (median 1 day). Of 581 patients with low bleeding risk procedures, for whom ≥24h preprocedural interruption is recommended, 188 (32.4%) had no interruption. Of 280 patients receiving high bleeding risk procedures, for whom ≥48h interruption is recommended, 46 (16.4%) had no interruption. Clinical events are shown in Table.
Conclusion: Edoxaban interruption schedules in routine practice were not always consistent with EHRA guidelines; nevertheless, rates of clinically significant bleeding or acute thromboembolic events or coronary syndromes were low in the EMIT study.
Colonna:Boehringer Ingelheim: Honoraria; Daiichi Sankyo: Honoraria; BayerAG: Honoraria; Italian Cardiology Association: Other: Non-financial support; European Society of Cardiology: Other: Non-financial support; Pfizer/BMS: Honoraria. von Heymann:Daiichi Sankyo: Honoraria, Research Funding; Boehringer Ingelheim: Honoraria; Bayer AG: Honoraria; Pfizer GmBH: Honoraria; CSL Behring: Honoraria; NovoNordisk Pharma: Honoraria; EACTA and EACTS: Other; DGGG: Other; Mitsubishi Pharma: Honoraria; Ferring GmbH: Honoraria; Biotest GmbH: Honoraria; Leo Pharma GmBH: Honoraria; German Society of Anaesthesiology and Intensive Care Medicine: Other. Saxena:Daiichi Sankyo: Honoraria, Research Funding. Vanassche:Boehringer Ingelheim: Honoraria; 367 Leo Pharma: Honoraria; Bayer: Honoraria; Daiichi Sankyo: Honoraria, Research Funding. Jin:Daiichi Sankyo: Current Employment. Wilkins:Daiichi Sankyo: Honoraria. Chen:Daiichi Sankyo: Current Employment. Unverdorben:Daiichi Sankyo: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal